What’s New in Progeria Research
 We’ve added this section so you can easily access information on the latest, important scientific publications on Progeria research.
We’ve added this section so you can easily access information on the latest, important scientific publications on Progeria research.
In addition to the articles highlighted below, there are now hundreds of articles on Progeria and Progeria-related subjects. We suggest you search PubMed to find the specific topic(s) you are looking for.
In an article published in Expert Opinion and authored by Executive Director Audrey Gordon and Medical Director Leslie Gordon, the two PRF leaders discuss PRF’s history, goals and accomplishments, and how the PRF programs have been pivotal in the journey from obscurity to treatment.
 The authors write, “It is our hope that the description of the PRF programs and services that follows, along with an account of how they are helping PRF accomplish its mission to save children with Progeria, will assist and inspire others to take similar action for the many rare disease populations that need immediate attention.”
The authors write, “It is our hope that the description of the PRF programs and services that follows, along with an account of how they are helping PRF accomplish its mission to save children with Progeria, will assist and inspire others to take similar action for the many rare disease populations that need immediate attention.”
This study demonstrates there is evidence that a farnesyltransferase inhibitor (FTI) can extend the lives of children with Progeria by at least one-and-a-half years. The study showed an extension of mean survival of 1.6 years during the six years following initiation of treatment. Two additional drugs added later in the trials, pravastatin and zoledronate, may also contribute to this finding. This is the first evidence of treatments influencing survival for this fatal disease.
Click here for more details.
Impact of Farnesylation Inhibitors on Survival in Hutchinson-Gilford Progeria Syndrome, Leslie B. Gordon, MD, PhD, Joe Massaro, PhD, Ralph B. D’Agostino Sr., PhD, Susan E. Campbell, MA, Joan Brazier, MS, W. Ted Brown, MD, PhD, Monica E Kleinman, MD, Mark W. Kieran MD, PhD and the Progeria Clinical Trials Collaborative; Circulation, May 2, 2014 (on-line).
The results of the first-ever clinical drug trial for children with Progeria reveal that Lonafarnib, a type of farnesyltransferase inhibitor (FTI) originally developed to treat cancer, has proven effective for Progeria. Every child showing improvement in one or more of four ways: gaining additional weight, better hearing, improved bone structure and/or, most importantly, increased flexibility of blood vessels. The study* was funded and coordinated by The Progeria Research Foundation.
Click here for more details.
*Gordon LB, Kleinman ME, Miller DT, Neuberg D, Giobbie-Hurder A, Gerhard-Herman M, Smoot L, Gordon CM, Cleveland R, Snyder BD, Fligor B, Bishop WR, Statkevich P, Regen A, Sonis A, Riley S, Ploski C, Correia A, Quinn N, Ullrich NJ, Nazarian A, Liang MG, Huh SY, Schwartzman A, Kieran MW, Clinical Trial of a Farnesyltransferase Inhibitor in Children with Hutchinson-Gilford Progeria Syndrome, Proceedings of the National Academy of Sciences, October 9, 2012 vol. 109 no. 41 16666-16671
Spanish and French scientists under the leadership of Carlos López-Otin (Oviedo) and Nicolas Lévy (Marseille) have published an exciting study that may result in a new approach to treating Progeria (1). While drugs used in PRF’s clinical trials to date have targeted changes made in the abnormal lamin A protein (progerin) that is made in Progeria cells, in the new work, the aberrant “splicing” of the lamin A messenger RNA (mRNA) coding for the lamin A protein is blocked, resulting in lowering the production of progerin. The blocking agent used is a small modified RNA molecule whose sequence is complementary to the region of the Progeria mRNA at which the splicing occurs. This molecule binds to the splice site and prevents the binding there of the complex of protein and RNA molecules required for splicing (the “spliceosome”).
That aberrant splicing in cultured skin cells of Progeria can be prevented in this manner was shown in 2005 (2). However, for treatment of patients the inhibiting reagent must be delivered intact to all tissues of the patient. it took another six years, and work in several laboratories, to develop these “delivery” methods.
In the new research (1), blocking the aberrant splicing in the model mouse resulted in impressive results. There were clear reductions in progerin concentrations in all tissues analyzed except skeletal muscle, which may have a reduced uptake of the blocking agent. The model mice recapitulated many of the phenotypes of Progeria patients, including
- Severely shortened life span (103 days compared to 2 years for wild-type mice.)
- Reduction of growth rate.
- Abnormal posture with curvature of the spine.
- Profound nuclear aberrations as a result of progerin accumulation.
- General loss of the fat layer under the skin.
- Profound bone alterations.
- Cardiovascular alterations, including significant loss of vascular smooth muscle cells.
- Alterations in the concentrations of various hormones in circulating blood plasma, including insulin and growth hormone.
The in vivo demonstration of the efficacy of reducing progerin production by blocking the aberrant splicing is a strong candidate for a valuable new approach to Progeria therapy.
(1) Osorio FG, Navarro CL, Cadiñanos J, López-Mejia IC, Quirós PM, et al, Science Translational Medicine, 3: Issue 106, advance on-line publication, October 26 (2011).
(2) Scaffidi, P. and Misteli, T. Reversal of the , cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome, Nature Medicine 11 (4): 440-445 (2005).
Researchers at the National Institutes of Health and Massachusetts General Hospital in Boston, MA published a new study today in Science, Translational Medicine that may lead to a new drug treatment for children with Progeria.*
Rapamycin is an FDA approved drug that has previously been shown to extend the lives of non-progeria mouse models. This new study demonstrates that rapamycin decreases the amount of the disease-causing protein progerin by 50%, improves the abnormal nuclear shape, and extends the lifespan of progeria cells. This study provides the first evidence that rapamycin may be able to decrease 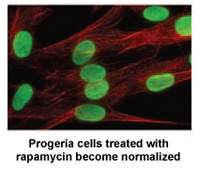 progerin’s damaging effects in children with progeria.
progerin’s damaging effects in children with progeria.
There is tremendous media coverage on this! Click below for links to media stories:
Wall Street Journal Health Blog
The Progeria Research Foundation was delighted to provide cells for this project from the PRF Cell & Tissue Bank, and help fund the research through our grants program.
This exciting new study demonstrates the remarkable pace of progeria research, while providing further insight into the aging process that affects us all.
*”Rapamycin Reverses Cellular Phenotypes and Enhances Mutant Protein Clearance in Hutchinson-Gilford Progeria Cells”
Kan Cao, John J. Graziotto, Cecilia D. Blair, Joseph R. Mazzulli, Michael R. Erdos, Dimitri Krainc, Francis S. Collins
Sci Transl Med. 2011 Jun 29;3(89):89ra58.
CBS Evening News, Wall Street Journal and Others Report on New Study
National Institutes of Health researchers have discovered a previously unknown link between Progeria and aging. The findings provide insights about the relationship between the toxic, Progeria-causing protein known as progerin and telomeres, which protect the ends of DNA within cells until they wear away over time and the cells die.
The study* appears in the June 13, 2011 early online edition of the Journal of Clinical Investigation. It concludes that in normal aging, short or dysfunctional telomeres stimulate cells to produce progerin, which is associated with age-related cell damage.
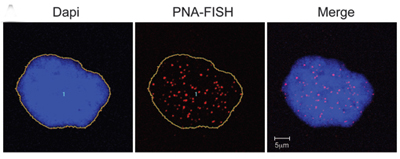 Progerin-expressing cells from normal individuals show signs of senescence. DNA in the nucleus is stained blue. Telomeres are seen as red dots. |
“For the first time, we know that telomere shortening and dysfunction influences the production of progerin,” says The Progeria Research Foundation Medical Director Leslie B. Gordon, MD, PhD. “Thus these two processes, both of which influence cellular aging, are actually linked.”
Prior research has shown that progerin is not only produced in children with Progeria, but that it is produced in smaller amounts in all of us, and progerin levels increase with aging. Independently, previous research on telomere shortening and dysfunction has been associated with normal aging. Since 2003, with the discovery of the Progeria gene mutation and the progerin protein that causes the disease, one of the key areas of research has focused on understanding whether and how Progeria and aging are linked.
“Connecting this rare disease phenomenon and normal aging is bearing fruit in an important way,” said NIH Director Francis S. Collins, MD, PhD, a senior author of the paper. “This study highlights that valuable biological insights are gained by studying rare genetic disorders such as Progeria. Our sense from the start was that Progeria had a lot to teach us about the normal aging process. “
Scientists have traditionally studied telomeres and progerin separately. While there is still much to learn about whether this new connection can lead to a cure for children with Progeria or potentially be applied to extending the human lifespan, this study provides further evidence that progerin, the toxic protein discovered through finding the gene mutation in Progeria, plays a role in the normal aging process.
*Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts, Cao et al, J Clin Invest doi:10.1172/JCI43578.
![]() Click here for the full text of the NIH press release.
Click here for the full text of the NIH press release.
A newly discovered gene mutation associated with a Progeria-like disease could open the door for possible new treatments for premature aging disorders and could provide fresh insight into normal aging.
A research team led by Progeria researcher Dr. Carlos López-Otín from the University of Oviedo in Spain encountered two families whose children have a previously unknown accelerated aging disease similar to Progeria. The children showed no defects in any genes that had previously been linked to progeroid diseases, but by studying the “coding” portions of their genomes, the team found a defect in a gene called BANF1. Family members with the progeroid disease had very low amounts of the protein made by BANF1, and, like people with Progeria, the nuclear envelopes in their cells were markedly abnormal. The abnormalities went away in cell culture experiments when the defective gene was replaced with the correct version. The findings were published in the American Journal of Human Genetics in May 2011.
BANF1 now joins the group of known genes that appear to influence some forms of premature aging—and that might affect normal aging as well.
In the past few years, scientists have been able to better understand normal aging on a molecular level thanks in part to studies of premature aging syndromes like this one as well as Progeria, which “cause the early development of characteristics normally associated with advanced age,” said López-Otín. He added that his study “underscores the importance of the nuclear lamina for human aging and demonstrates the utility of the new methods of genome sequencing to identify the genetic cause of rare and devastating diseases, which have traditionally received limited attention.”
Xose S. Puente, Victor Quesada, Fernando G. Osorio, Rubén Cabanillas, Juan Cadiñanos, Julia M. Fraile, Gonzalo R. Ordóñez, Diana A. Puente, Ana Gutiérrez-Fernández, Miriam Fanjul-Fernández et al. “Exome Sequencing and Functional Analysis Identifies BANF1 Mutation as the Cause of a Hereditary Progeroid Syndrome.” American Journal of Human Genetics, May 5, 2011 DOI: 10.1016/j.ajhg.2011.04.010
In recent years, the factors that regulate longevity have been extensively studied. Signaling between growth hormone(GH) and insulin-like growth factor 1 (IGF-1) has been identified as a major regulator in animals ranging from worms to man. (This signaling system is often referred to as the somatotrophic axis.) In this article, Dr. Carlos López-Otin and his colleagues at the University of Oviedo (Spain), studied whether, and how, changes in these hormones play a role in premature aging in a progeric mouse model, Zmpste 24-/-.. IGF-1 hormone levels decreased and growth hormone levels increased when the mice aged. Growth hormone is known to be the major regulator of IGF-1. These hormone changes did not happen in the normal mice, which tells us that the changes are a consequence of disease in the progeroid mice.
The investigators treated the progeroid mice with IGF-1 and found substantial recovery from progeroid phenotypes, including weight gain, increased amount of subcutaneous fat, reduced kyphosis (curvature of the spine) and alopecia (baldness), and increased lifespan, with a 17% extension of the median life span (from 123 days to 145 days) and a 24% increase in the maximum lifespan (from 151 days to 187 days).
These findings highlight that levels of the hormones insulin-like growth factor 1 and growth hormone are important in controlling longevity in this mouse model of Progeria. Unlike this mouse, children with Progeria have normal levels of IGF-1 and growth hormone. Given the links between Progeria and aging, this study may lead to additional approaches to therapy of HGPS, as the mechanisms by which these hormones affect longevity are discovered.
Mariño G, Ugalde AP, Fernández AF, Osorio FG, Fueyo A, Freije JM, López-Otín C. “Insulin-like growth factor 1 treatment extends longevity in a mouse model of human premature aging by restoring somatotroph axis function.” Proc Natl Acad Sci U S A. 2010 Aug 30.
On August 26, 2010, Arteriosclerosis, Thrombosis, and Vascular Biology electronically published, ahead of print, the results of a study comparing Progeria and typical cardiovascular aging, entitled “Cardiovascular Pathology in Hutchinson-Gilford Progeria: Correlation With the Vascular Pathology of Aging”. The study found that progerin, the abnormal protein that causes Progeria, is also present in the vasculature of the general population and increases with age, adding to the growing case that there are parallels between normal aging and progeria aging.
Researchers examined cardiovascular autopsies and progerin distribution in patients with Progeria along with a group without Progeria between the ages of one month and 97 years, and found that progerin in individuals without Progeria increased an average of 3.3 percent per year in the coronary arteries.
“We found similarities between many aspects of cardiovascular disease in both Progeria and the atherosclerosis that affects millions of people throughout the world” said Dr. Leslie Gordon, senior author of the study and The Progeria Research Foundation’s Medical Director. “By examining one of the rarest diseases in the world, we are gaining crucial insight into a disease that affects millions of people worldwide. Ongoing research has the potential to have a significant impact on our understanding of heart disease and aging.”
This study supports the possibility that progerin is a contributor to the risk of atherosclerosis in the general population, and merits examination as a potential new trait to help predict heart-disease risk.
Olive M, Harten I, Mitchell R, Beers J, Djabali K, Cao K, Erdos MR, Blair C, Funke B, Smoot L, Gerhard-Herman M, Machan JT, Kutys R, Virmani R, Collins FS, Wight TN, Nabel EG, Gordon LB.
“Cardiovascular Pathology in Hutchinson-Gilford Progeria: Correlation With the Vascular Pathology of Aging”. Arterioscler Thromb Vasc Biol. 2010 Nov;30(11):2301-9; Epub 2010 Aug 26.
Click here for the full press release.
In this article, Catherine Shanahan and her group at Oxford University have made a major advance in elucidating a key step in the aging of human blood vessels (vascular aging.) The experiments derive directly from work on Progeria, performed in a number of laboratories. The Shanahan group’s two key findings are: (1) prelamin A accumulates in vascular smooth muscle cells (VSMCs) of aged individuals but not of young individuals, and (2) this accumulation results, at least in part, from depletion of the enzyme FACE1. FACE1(also called Zmpte24) is required for the removal of the farnesyl group in prelamin A, during processing to normal lamin A, a critical component of the cell nucleus.
This situation is very similar to that in Progeria. There, prelamin A (called progerin) retains the farnesyl group. Indeed, the initial step in causing the disease is the failure to remove the farnesyl group. This failure happens because the Progeria mutation results in deletion of the part of prelamin A needed for FACE 1 to bind and remove the farnesyl group. Thus, the cause of the defects in aging and Progeria are the same: FACE1 can not do its job.
It has been known for some years that farnesyl transferase inhibitors (FTIs) inhibit (and can reverse) the presence of nuclear markers of disease in Progeria cells. Now, Shanahan et al have found that FTIs inhibit the appearance of similar nuclear markers in cells from aged normal individuals. FTIs are currently in use in Progeria clinical trials and Shanahan et al note that, these clinical trials “will shed further light on the therapeutic potential of these drugs in the treatment of aging.”
The studies described in this article are the best example to date of how studies of Progeria are furthering our understanding of normal aging.
Ragnauth CD, Warren DT, Liu Y, Shanahan CM et al, “Prelamin A Acts to Accelerate Smooth Muscle Cell Senescence and is a Novel biomarker of Human Vascular Aging.” Circulation: May 25, 2010, pp. 2200-2210.
In our February posting of “What’s New in Progeria Research” we reported evidence that a farnesyl transferase inhibitor (FTI) acts to relieve disease symptoms by the farnesylation of progerin, and not by inhibiting proteins other than progerin. The UCLA group headed by former PRF research grantees Stephen Young and Loren Fong has now reported results with another severe progeroid laminopathy that support this conclusion. In Restrictive Dermatopathy (RD), the prelamin A remains farnesylated, as is the case for progerin in Progeria patients, RD prelamin A does not have the 50 amino acid deletion of progerin, but it has retained the terminal 15 amino acids at the carboxyl end of prelamin A, which is cleaved off in progerin.
Davies and coworkers prepared a new model mouse whose prelamin A, unlike RD prelamin A, is not farnesylated, but does retain the 15 amino acid sequence that is normally cleaved in the path to synthesize lamin A. This mouse does not have progeroid symptoms, indicating that in RD, as well as in Progeria, the presence of the farnesyl group, and not a change in amino acid sequence, is responsible for the disease symptoms.
DaviesBS, Barnes RH 2nd, Tu Y, Ren S, Andres DA, Spielmann HP, Lammerding J, Wang Y, Young SG, Fong LG,
“An accumulation of nonfarnesylated prelamin A causes cardiomyopathy but not progeria”, Hum Mol Genet. 2010 Apr 26. [Epub ahead of print]
 The authors evaluated the possibility that the ameliation of progeroid disease by a farnesyltransferase inhibitor (FTI) in a mouse model of Progeria is due to the effect of the drug on farnesylation of protein(s) other than progerin. They constructed a mouse that made unfarnesylated progerin, but not farnesylated progerin. This mouse also developed progeria-like disease phenotypes, but FTI did not ameliorate them. This result indicates that the drug does not act by inhibiting proteins other than progerin; it must be acting on the farnesylation of progerin, the biochemical step that is not present in the tested model.
The authors evaluated the possibility that the ameliation of progeroid disease by a farnesyltransferase inhibitor (FTI) in a mouse model of Progeria is due to the effect of the drug on farnesylation of protein(s) other than progerin. They constructed a mouse that made unfarnesylated progerin, but not farnesylated progerin. This mouse also developed progeria-like disease phenotypes, but FTI did not ameliorate them. This result indicates that the drug does not act by inhibiting proteins other than progerin; it must be acting on the farnesylation of progerin, the biochemical step that is not present in the tested model.
Yang SH, Chang SY, Andres DA, Spielmann HP, Young SG, Fong LG. “Assessing the efficacy of protein farnesyltransferase inhibitors in mouse models of progeria.”
J Lipid Res. 2010 Feb;51(2):400-5. Epub 2009 Oct 26.
In 1921, F. Scott Fitzgerald published a short story entitled ‘The Curious Case of Benjamin Button’, which was made into a movie in 2008 starring Brad Pitt. The main character of Fitzgerald’s fictional work is born with a very rare condition in which he looks like an elderly person. The main difference between the fictional individual and individuals with HGPS is that Fitzgerald’s character becomes younger as the years go by. This paper scientifically presents the possibility that Fitzgerald consciously based his character, Benjamin Button, upon individuals with HGPS, and that HGPS individuals might not only have the appearance of an aged person, but also might actually undergo true physical aging, which would enable researchers to gain valuable information into the treatment of ailments commonly associated with the natural process of aging.
Maloney WJ, “Hutchinson-Gilford Progeria syndrome: its presentation in F. Scott Fitzgerald’s short story ‘the curious case of Benjamin Button’ and its oral manifestations.”
J. Dent. Res 2009 Oct 88 (10): 873-6
HGPS has previously been shown to affect many fundamental cellular functions including replication, gene expression, and DNA repair. Busch and coworkers have added the transport of proteins from the cytoplasm into the nucleus to this list. All proteins are synthesized in the cytoplasm, and those that end up being in the nucleus have to get across the nuclear membrane. The transport is accomplished through channels in the nuclear membrane called “nuclear pores”. Many proteins are too large to simply diffuse through the nuclear pores, but are “ushered” through them by special proteins that have evolved for this purpose. In this article, cells that express the mutant gene responsible for HGPS were found to have reduced transport of proteins into nuclei by direct measurement.
Busch A, Kiel T, Heupel WM, Wehnert M, Huebner S., “Nuclear protein import is reduced in cells expressing nuclear envelopathy-causing lamin A mutants.” Exp Cell Res. 2009 May 11.
This article is a very thoughtful and up-to-date review which will be of interest to investigators working on progeroid diseases (with emphasis on HGPS) and their relation to normal aging, It also touches on the relation of aging to cancer. Topics covered are:
→ Providing structure and organization: nuclear architecture and genome integrity
→ DNA damage and repair gone awry
→ Old and beyond repair tumor suppressors and cellular senescence, and
→ Regeneration and renewal: stem-cell biology. Regeneration and renewal: stem-cell biology.
The article highlights the ways in which recent advances in the study of progeroid diseases is giving insight into basic cellular functions as well as aging.
Capell BS, Tlougan BE, Orlow SJ, “From the Rarest to the Most Common: Insights from Progeroid Syndromes into Skin Cancer and Aging.” Journal of Investigative Dermatology (2009 Apr 23), 1-11
Previous experiments with Fibroblast cells from Progeria patients have shown that the damage caused by the mutation is initially the result of action by the altered form of Lamin A, called Progerin. But the interpretation of these experiments can be difficult in culture for varying numbers of generations. Fong et. al. have set up an experimental system in which the amount of Progerin in Wild-type cells can be increased or decreased. This method will allow investigators to sort out the direct effects of Progerin from secondary ones, thereby advancing the study of cellular mechanisms that lead to the pathophysiology of Progeria cells.
Activating the synthesis of progerin, the mutant prelamin A in Hutchinson-Gilford progeria syndrome, with antisense oligonucleotides. (PubMed Article) Fong LG, Vickers TA, Farber EA, Choi C, Yun UJ, Hu Y, Yang SH, Coffinier C, Lee R, Yin L, Davies BS, Andres DA, Spielmann HP, Bennett CF, Young SG , “Activating the synthesis of progerin, the mutant prelamin A in Hutchinson-Gilford progeria syndrome, with antisense oligonucleotides.” Hum Mol Genet. 2009 Apr 17.
Drs. Fong and Young have previously been funded with grants from The Progeria Research Foundation.
Swedish Team Finds a Build-up of Progerin RNA in Normal Cells as They Age
Progerin is the abnormal protein causing Progeria. In recent years, several research groups have found that normal cells also produce progerin, but much less than the cells of a child with Progeria. Moreover, the amount of progerin protein in normal cells increases as they age in the laboratory. These results established a direct link at the cellular level between Progeria and normal aging.
Dr. Maria Eriksson, author of the gene finding for Progeria in 2003, has now invented a new, powerful technique to quantitatively measure the expression of the Progeria gene. Dr. Eriksson’s laboratory at the Karolinska Institute in Sweden used the technique to measure the amount of progerin RNA in both normal and Progeria cells. RNA is the blueprint molecule in our cells for making protein. The Swedish group found that both normal and Progeria cells make larger and larger amounts of progerin RNA as they age. Eriksson’s result shows that the RNA signal for making progerin quickly builds in the cells of children with Progeria, and builds slowly over a lifetime in us all.
These new findings strengthen our understanding of the connection between normal aging and Progeria. In addition, the new technique is expected to be widely used in experiments that address the mechanism of progerin action.
Rodriguez S, Coppedè F, Sagelius H and Erikson M. “Increased expression of the Hutchinson-Gilford progeria syndrome truncated lamin A transcript during cell aging”. European Journal of Human Genetics (2009), 1-10.
August and October 2008: Two separate studies show that Progeria is reversible in the cardiovascular system and the skin of mouse models. The experiments were significant in not treating the mice until they expressed Progeria symptoms, whereas most previous studies began treatment before Progeria was apparent. Production of progerin (the damaging protein made from the Progeria gene) was inhibited either by treatment with a farnesyl transferase inhibitor (FTI) or by turning off the gene. In both cases the mice reverted to normal or almost normal conditions. These observations provide encouraging evidence for the current clinical trial of FTIs for Progeria.
In a stunning display of progress with the FTI drug – now being used in the First-ever Progeria Clinical Drug Trial – Dr. Francis Collins’ research team at the National Institutes of Health * found that FTI’s prevented and even reversed the most devastating effect of Progeria in mice: cardiovascular disease.* “We were amazed that [the drug] worked so well,” says Francis Collins, a geneticist and former director of the National Human Genome Research Institute, who was senior author for the research team that identified the Progeria gene mutation in 2003. “Not only did this drug prevent these mice from developing cardiovascular disease, it reversed damage in mice that already had disease.”
The Progeria mice develop heart disease that mirrors that of children with Progeria. The authors found that the FTI was both able to prevent the development of heart disease to some degree when mice were treating from the time they were weaned, and partially reverse established disease when mice were treated beginning at age 9 months. “One of the striking things from my perspective was the ability to reverse disease, ” Collins said, which is critical given that Progeria is generally not diagnosed at birth, but only when children begin to show symptoms, when part of the damage already has been done.
“If these drugs are found to have similar effects in children, this could mark a major breakthrough for treating this devastating disease,” said NHLBI’s Dr. Nabel, who was a co-author of the study. “In addition, these findings shed light on the potential role of FTI drugs to treat other forms of coronary artery disease.”
View the article in Scientific American, “New Hope for Progeria: Drug for Rare Aging Disease”, at http://www.sciam.com/article.cfm?id=new-hope-for-progeria-drug-for-rare-aging-disease and the NIH press release at http://www.nih.gov/news/health/oct2008/nhgri-06.htm
* Capell, et. al, “A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a Progeria mouse model.” Proceedings of the National Academy of Sciences, Vol. 105, no. 41, 15902-15907 (Oct. 14, 2008)
In a second study that was published online in the Journal of Medical Genetics**, Dr. Maria Eriksson’s research team at the Karolinska Institutet in Sweden created another mouse model of Progeria with abnormalities of the skin and teeth. The mice are genetically engineered so that the Progeria mutation can be shut off at any time. Once disease was apparent, the gene for Progeria was turned off. After 13 weeks the skin was almost indistinguishable from normal skin. This study shows that in these tissues the expression of the Progeria mutation does not cause irreversible damage and that the reversal of disease is possible, which gives promise for treatment for Progeria.
**Eriksson, et. al., “Reversible phenotype in a mouse model of Hutchinson-Gilford Progeria syndrome.” J. Med. Genet. published online 15 Aug 2008; doi:10.1136/jmg.2008.060772
To purchase this article, go to: http://jmg.bmj.com/cgi/rapidpdf/jmg.2008.060772v1
More Evidence of the Link between Progeria and Normal Aging and Heart Disease
These exciting Capell and Eriksson studies show that beyond Progeria, these results have the potential to benefit all patients with cardiovascular disease. Researchers have discovered that the toxic protein responsible for Progeria is actually produced at low levels in all humans, possibly accumulating as we age. Thus, by studying these rare children, we can further our understanding of a major mechanism of human aging—and perhaps, find new ways to slow the process.
Here are some past headlines on Progeria research milestones that have helped advance the field at a fantastic pace:
2007:
2007 International Progeria Workshop Featured in Journal of Gerontology
PRF-Funded Studies Provide Support for Drug Trial
2006:
Research Suggests Link Between Progeria and Normal Aging
2005:
Exciting News on Potential Drug Treatments
Blocking Protein May Prove Useful in Treating Progeria
Reversal of the cellular phenotype in the premature aging disease HGPS
2004:
Gene Mutation Causes Progressive Changes to Cell Structure in Children with Progeria
2003:
Identification of Gene Gives Hope to Children with Progeria Progeria Gene Discovered
