October 2008: First-Ever Progeria Clinical Drug Trial Surpasses Half-Way Mark!
PRF continues to make history, as nearly all the children enrolled in the trial have come to Children’s Hospital Boston for their 1-year visit, marking their half-way point to completion. Click here for details on how you can help.
Exciting times! The Progeria clinical drug trial began on May 7th, 2007 with two children arriving in Boston, MA for their first of seven visits over a 2-year period. At this first visit, they were given extensive tests and their first doses of the drug. An average of two families have been flying to Boston each week since then, and in October 2007, the trial became fully enrolled. The trial is expected to end in October 2009, with results published in 2010.
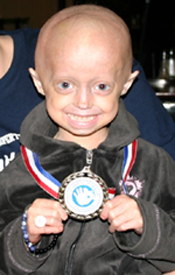
Megan proudly wears her 1-year Trial Medal, which she received at the end of her recent trip to Boston
“I know of no other rare genetic disease that has gone from gene discovery to clinical trial in under four years – a phenomenal testament to the hard work of The Progeria Research Foundation.”
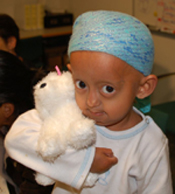
Julieta, from Argentina.
Francis Collins, MD, PhD, Director of the National Human Genome Research Institute that mapped the human genome, workshop speaker and co-discoverer of the Progeria gene.
- Argentina
- Belgium
- Canada
- Denmark
- England
- India
- Israel
- Italy
- Japan
- Mexico
- Pakistan
- Poland
- Portugal
- Romania
- USA
- Venezuela

“The two Megans”, both 6 years old, in Boston for the clinical trial

Michiel, 8 ½ , from Belgium with Hayley, 9 ½ , from England in June at Children’s Hospital Boston during their first visit.
The Progeria Clinical Research Drug Trial: Who, Where, When, How and How Much…
The clinical trial is led by Mark Kieran MD, PhD, Director, Pediatric Medical Neuro-Oncology, Dana-Farber Cancer Institute and Children’s Hospital Boston; Assistant Professor, Departments of Pediatrics and Hematology/Oncology, Harvard Medical School. Dr. Kieran is a pediatric oncologist with extensive experience with the drug under study (farnesyltransferase, or FTI) in children.
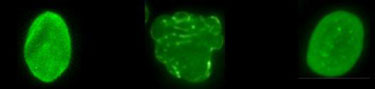
Progeria cells become normalized when FTIs are applied. Capell et al., PNAS, 2005. Normal cell. Progeria cell. Progeria cell after being treated with FTI
 during that 2 ½ year time period.
during that 2 ½ year time period.PRF needs to raise approximately $2 million dollars to fund this trial, and as of July 2009, we have raised $1.9 million!

Our Circle of Hope has expanded…

Recent Comments